A part of a homologous series is shown below.
Which of these compounds is a part of the series shown above?

Important Questions on Carbon and its Compounds
The picture shows the bonds between atoms in two allotropes of carbon.
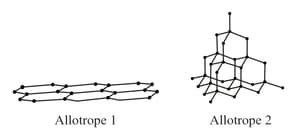
Which allotrope is harder? Explain your answer.
On undergoing complete combustion in an adequate supply of oxygen, an organic compound produces only carbon dioxide and water vapour as the products.
Based on this information, Which of the following homologous series could the compound belong to?
alkanes
alcohols
aldehydes
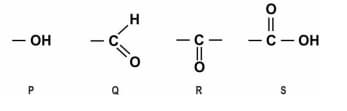
A compound with which of the following functional groups is most likely to cause the decomposition of baking soda to produce carbon dioxide?
Which of the following will be different for the two reactions?
The number of moles of the saturated compound formed.
The number of moles of the hydrogen consumed.
Kohli has three test tubes containing hexane, hexene and hexyne respectively. Which of the three compounds can he identify using the bromine water test? Give a reason for your answer.
A carbon compound of molecular formula contains a ketone functional group. Draw the structures of three isomers of this compound having a ketone group.
Heating an alcohol with concentrated sulphuric acid results in the dehydration of the alcohol to give the alkene as shown by the reaction of ethanol to give ethene.
Pramila heated -butanol (shown below) with concentrated sulphuric acid.
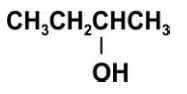
Write the structural formulae of all the possible products of the reaction.
An alkane has carbon atoms arranged within ring structures as shown below:
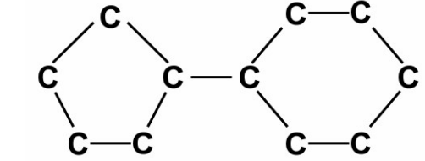
What is the molecular formula of alkane?
