A particular atom of carbon () is represented like this:
State the value of its proton number Z _____

Important Questions on The Nuclear Atom
A particular atom of carbon () is represented like this:
State the value of its nucleon number A _____
A particular atom of carbon () is represented like this:
Calculate the value of its neutron number N _____
Complete the table by identifying the subatomic particles described.
| Description | Which Particle |
| These particles make up the nucleus (there are two answers). | |
| These particles orbit the nucleus. | |
| These particles have very little mass. | |
| These particles have no electric charge. | |
| The charge on these particles is equal and opposite to the charge on an electron. |
The figure given below represents an atom of an isotope of boron (B).
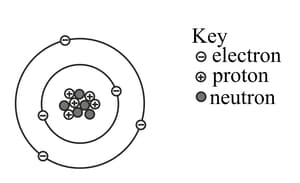
Write down the symbol for this nuclide in the form of .
The table shows some values of Z, N and A for six different nuclides. Complete the table as follows: Fill in the missing values of Z, N and A in the second, third, and fourth columns. Use the Periodic Table to identify the elements, and write your answer in the fifth column. Finally, in the last column write the symbol for each nuclide in the form .
| Nuclide | Proton number | Neutron number | Nucleon number | Name of Element | Nuclide Symbol |
| Nu-1 | |||||
| Nu-2 | |||||
| Nu-3 | |||||
| Nu-4 | |||||
| Nu-5 |
