EASY
MHT-CET
IMPORTANT
Earn 100
A photon is emitted by a hydrogen atom when it comes from excited state to the ground state. The recoil speed is almost
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atoms, Molecules and Nuclei
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
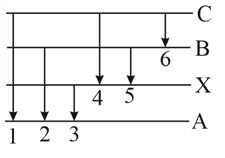
In the figure, six lines of emission spectrum are shown. Which of them will be absent in the absorption spectrum?
EASY
MHT-CET
IMPORTANT
