MEDIUM
NEET
IMPORTANT
Earn 100
A piece of is kept inside a balloon filled with some air, having thermally insulated walls. The temperature is kept just above sublimation temperature of .
.
Which of the following is correct regarding this?
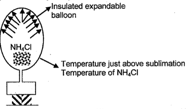

(a) positive
(b) negative
(c) zero
(d) positive
100% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
HARD
NEET
IMPORTANT
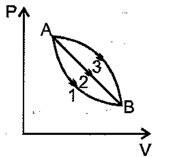
A given mass of gas expands from the state to the state by three paths and as shown in the figure. If and , respectively be the magnitudes work done by the gas alonq three paths then
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
