A poly atomic molecule has translational, rotational degrees of freedom and vibrational modes. The ratio of specific heats is
Important Questions on Kinetic Theory of Gases
Match the ratio for ideal gases with different type of molecules:
| Molecule Type | |
| (A) Monoatomic | (I) |
| (B) Diatomic rigid molecules | (II) |
| (C) Diatomic non-rigid molecules | (III) |
| (D) Triatomic rigid molecules | (IV) |
Given below are two statements:
Statement I: In a diatomic molecule, the rotational energy at a given temperature obeys Maxwell's distribution.
Statement II : In a diatomic molecule, the rotational energy at a given temperature equals the translational kinetic energy for each molecule.
In the light of the above statements, choose the correct answer from the options given below:
Using equipartition of energy, the specific heat (in ) of Aluminium at high temperature can be estimated to be (atomic weight of Aluminium )
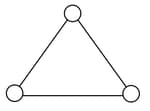
Consider a gas of triatomic molecules. The molecules are assumed to be triangular and made of massless rigid rods whose vertices are occupied by atoms. The internal energy of a mole of the gas at temperature T is:
(R-Universal gas constant)
Which statements are correct about degrees of freedom?
A. A molecule with degrees of freedom has different ways of storing energy.
B. Each degree of freedom is associated with average energy per mole.
C. A monoatomic gas molecule has rotational degree of freedom where as diatomic molecule has rotational degrees of freedom
D. has a total to degrees of freedom.
Choose the correct answer from the option given below:

