EASY
Earn 100
A proton and an alpha particle are accelerated in a field of the same potential difference. Then, the ration of the Brigle wavelengths associated with the moving material particles is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Atomic Physics
EASY
Scattering angle
Number of scattered -particles detected
(Plots are schematic and not to scale)
MEDIUM
EASY
Experimentally it is found that energy is required to separate a hydrogen atom into a proton and an electron. So the orbital radius of the electron in a hydrogen atom is .
The value of the is: _____.
( and electronic charge )
EASY
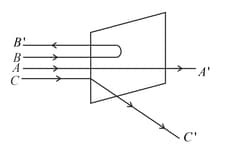
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
MEDIUM
EASY
HARD
HARD
EASY
EASY
EASY
EASY
(Ignore radiation due to motion of electric charge)
MEDIUM
EASY
HARD
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and

