MEDIUM
NEET
IMPORTANT
Earn 100
A quantity of air at is compressed suddenly, the temperature of the air system will :
(a)Fall
(b)Rise
(c)Remain unchanged
(d)First rise and then fall
100% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
Graphs between P-V diagram for isothermal and adiabatic processes are drawn. The relation between their slopes will be :-
EASY
NEET
IMPORTANT
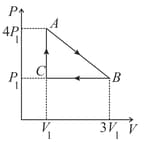
An ideal gas is taken round the cycle ABCA. In the cycle the amount of work done involved is:-
EASY
NEET
IMPORTANT
One mole ideal gas is compressed adiabatically at 27ºC. Its temperature becomes 102ºC. The work done in this process will be :- ( = 1.5)
EASY
NEET
IMPORTANT
The volume of a gas expands by 0.25 m3 at a constant pressure of 103 Nm-2. The work done is equal to (magnitude):
EASY
NEET
IMPORTANT
The process in which the heat given to a system is completely transformed into work is for ideal gas :-
EASY
NEET
IMPORTANT
The volume of a poly-atomic gas compressed adiabatically to of the original volume. If the original pressure of the gas is P0 the new pressure will be:
EASY
NEET
IMPORTANT
In an adiabatic process the quantity which remains constant is :-
EASY
NEET
IMPORTANT
calories of heat is supplied to raise the temperature of of air from to without any change in its volume. The change in internal energy per gram of air is,
