HARD
Earn 100
A radioactive isotope, A undergoes simultaneous decay to different nuclei as :
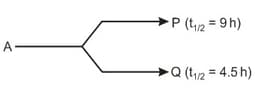
Assuming that initially neither P nor Q was present, after how many hours, amount of Q will be just double to the amount of A remaining ?
(a)6.0 h
(b)7.0 h
(c)8.0 h
(d)5.0 h
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
product
| Experiment | Initial rate of reaction | ||
The time (in minutes) required to consume half of is
EASY
HARD
MEDIUM
HARD
EASY
MEDIUM
EASY
MEDIUM
| [A] | [B] | [C] | Rate |
HARD
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

EASY
MEDIUM
Which one of the following statements is correct?
HARD
HARD
For an elementary chemical reaction, , the expression for is:
HARD
(Assume that all these gases behave as ideal gases)
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
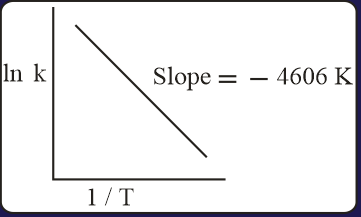
MEDIUM
MEDIUM
EASY

