A reaction between ammonia and boron trifluoride is given below:
Identify the acid and base in this reaction. Which theory explains it? What is the hybridisation of and in the reactants?

Important Questions on Equilibrium
The following data is given for the reaction:
Predict the effect of temperature on the equilibrium constant of the above reaction.
Match the following equilibria with the corresponding condition
| (i) | LiquidVapour | (a) | Saturated solution |
| (ii) | SolidLiquid | (b) | Boiling point |
| (iii) | Solid Vapour | (c) | Sublimation point |
| (iv) | Solute (s)Solute (solution) | (d) | Melting point |
| (e) | Unsaturated solution |
For the reaction :
Equilibrium constant
Some reactions are written below in Column I and their equilibrium constants in terms of are written in Column II. Match the following reactions with the corresponding equilibrium constant.
| Column I (Reaction) | Column II (Equilibrium constant) | ||
| (i) | (a) | ||
| (ii) | (b) | ||
| (iii) | (c) | ||
| (d) | |||
Match standard free energy of the reaction with the corresponding equilibrium constant
| Column I | Column II | ||
| (i) | (a) | ||
| (ii) | (b) | ||
| (iii) | (c) | ||
| (d) |
Match the following species with the corresponding conjugate acid
| Species | Conjugate acid | ||
|
(i) |
(p) | ||
| (ii) | (q) | ||
| (iii) | (r) | ||
| (iv) | (s) | ||
| (t) | |||
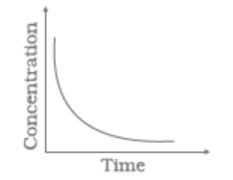
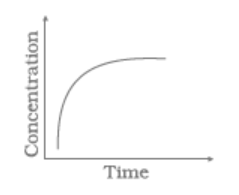
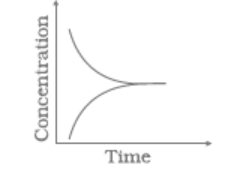
Match the following graphical variation with their description
| A | B | ||
| (i) |
|
(a) | Variation in product concentration with time |
| (ii) |
|
(b) | Reaction at equilibrium |
| (iii) |
|
(c) | Variation in reactant concentration with time |
Match Column (I) with Column (II).
| Column I | Column II | ||
| (i) | Equilibrium | (a) | |
| (ii) | Spontaneous reaction | (b) | |
| (iii) | Non-spontaneous reaction | (c) | |
| (d) | |||
How can you predict the following stage of a reaction by comparing the value of and ?
Net reaction proceeds in the forward direction.