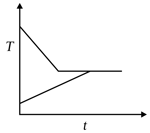
A sample A of liquid water and a sample of ice of identical mass are kept in two neighouring chambers in an otherwise insulated container. The chambers can exchange heat with each other. The graph of temperature of the two chambers is plotted with time.

Important Questions on Thermal Properties of Matter
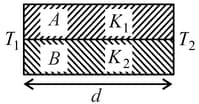
Two rods A and B of different materials are welded together as shown in figure. Their thermal conductivities are and . The thermal conductivity of the composite rod will be
Consider a ball of mass attached to one end of a spring and immersed in water. Assume the complete system is in thermal equilibrium. The spring is now stretched to and the mass is released so that it vibrates up and down. Estimate the change in temperature of water before the vibrations stop.
(Specific heat of the material of the ball and Specific heat of water )
The vapour pressure vs. temperature curve for a solution solvent system is shown below.
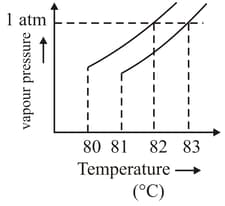
The boiling point of the solvent is _____°C.
The electrical resistance in ohms of a certain thermometer varies with temperature according to the approximate law:
The resistance is at the triple-point of water , and at the normal melting point of lead . What is the temperature when the resistance is ?
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
Two similar thermocouples made up of similar metals A and B are connected through a key K and sensitive galvanometer G as shown in the figure. One of the thermocouple is dipped in a hot bath maintained at temperature t2 and other one in a cold bath at a temperature t1. When the key K is pressed a deflection is seen in the galvanometer because:
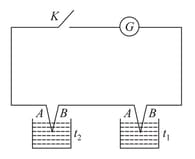
At temperature , the value of thermo electric power will be,
The pair of boiling point and compound are given as,
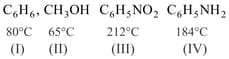
Which will show lowest vapour pressure at room temperature?
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
| Temperature (0oC) |
Vapour pressure of benzene (torr) |
Vapour pressure of chlorobenznee (torr) |
|---|---|---|
| 80 | 750 | 120 |
| 90 | 1000 | 200 |
| 100 | 1350 | 300 |
| 110 | 1800 | 400 |
| 120 | 2200 | 540 |
On an X temperature scale, water freezes at –125.0° X and boils at 375.0° X. On a Y temperature scale, water freezes at –70.0° Y and boils at –30.0° Y. The value of temperature on X-scale equal to the temperature of 50.0°Y on Y-scale is

