HARD
JEE Main
IMPORTANT
Earn 100
A sample of of an organic compound when analysed by Duma's method yields of nitrogen gas collected over solution at and . The percentage of nitrogen in the given organic compound is____
(a) The vapour pressure of water at is
(b)
50% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
HARD
JEE Main
IMPORTANT
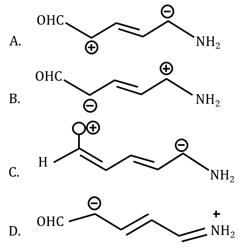
Increasing order of stability of the resonance structure is:
HARD
JEE Main
IMPORTANT
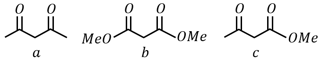
Which will undergo deprotonation most readily in basic medium?
EASY
JEE Main
IMPORTANT
Which of the following conformations will be the most stable?
EASY
JEE Main
IMPORTANT
Match List I with List II.
Choose the correct answer from the options given below :-
| List I Isomeric pairs |
List II Type of isomers |
||
| A | Propanamine and N- Methylethanamine |
I | Metamers |
| B | Hexan-2-one Hexan-3-one |
II | Positional isomers |
| C | Ethanamide and Hydroxyethanimine |
III | Functional isomers |
| D | o-nitrophenol and pnitrophenol | IV | Tautomers |
MEDIUM
JEE Main
IMPORTANT
Compound that will give positive Lassaigne's test for both nitrogen and halogen is
MEDIUM
JEE Main
IMPORTANT
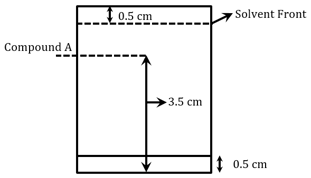
Following chromatogram was developed by adsorption of compound '' on a TLC glass plate. Retardation factor of the compound '' is _____ .
MEDIUM
JEE Main
IMPORTANT
When of an organic compound containing carbon was burnt completely, of was produced. The molar mass of compound is _____ (Nearest integer)
HARD
JEE Main
IMPORTANT
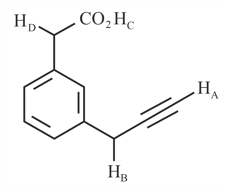
What is the correct order of acidity of the protons marked in the given compounds?