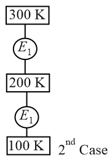
A sample of an ideal gas is taken through the cyclic process as shown in figure. It absorbs, of heat during the part , no heat during and rejects of heat during . A work of is done on the gas during the part . The internal energy of the gas at is . The work done by the gas during the part is


Important Questions on Thermodynamics
(Given )
Read the following statements :
. When small temperature difference between a liquid and its surrounding is doubled the rate of loss of heat of the liquid becomes twice.
. Two bodies and having equal surface areas are maintained at temperature and . The thermal radiation emitted in a given time by and are in the ratio
. A carnot Engine working between and has an efficiency of
. When small temperature difference between a liquid and its surrounding is quadrupled, the rate of loss of heat of the liquid becomes twice.
Choose the correct answer from the options given below :
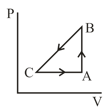
In case, Carnot engine operates between temperatures and . In case, as shown in the figure, a combination of two engines is used. The efficiency of this combination (in case) will be :