EASY
AS and A Level
IMPORTANT
Earn 100
A sample of boron was found to have the following composition by mass: , . Calculate a value for the relative atomic mass of boron. Give your answer to significant figures.

Important Questions on Atoms, Molecules and Stoichiometry
EASY
AS and A Level
IMPORTANT
Boron ions, , can be formed by bombarding gaseous boron with high-energy electrons. Deduce the number of electrons in one ion.
EASY
AS and A Level
IMPORTANT
Boron is present in compounds called borates. Use the values below to calculate the relative molecular mass of iron(III) borate, .
( values: ).
EASY
AS and A Level
IMPORTANT
Boron is present in compounds called borates. The accurate relative atomic mass of iron, , is . Explain why the accurate relative atomic mass is not a whole number.
EASY
AS and A Level
IMPORTANT
This question is about some metals and metal compounds. Hafnium, , forms a hydrated peroxide whose formula can be written as . Use the values below to calculate the relative molecular mass of hydrated hafnium peroxide. ( values: ).
EASY
AS and A Level
IMPORTANT
What is meant by the term hydrated salt?
EASY
AS and A Level
IMPORTANT
A particular isotope of hafnium has protons and a nucleon number of . Write the isotopic symbol for this isotope, showing this information.
EASY
AS and A Level
IMPORTANT
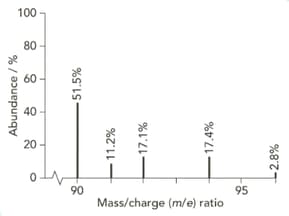
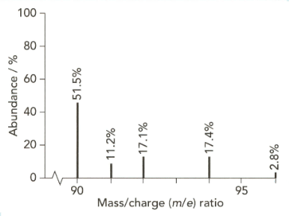
The mass spectrum of zirconium is shown below.
Give the isotopic symbol for the most abundant isotope of zirconium.
EASY
AS and A Level
IMPORTANT
The mass spectrum of zirconium is shown below.
Use the information from this mass spectrum to calculate the relative atomic mass of zirconium. Give your answer to significant figures.