A simple mechanism for enzyme-catalyzed reaction is given by the following set of equations:
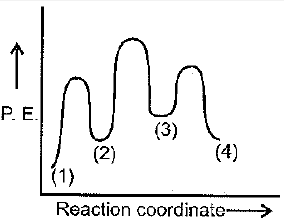
This is known as the Michaelis-Menten mechanism. The potential energy diagram is shown in the figure. Which of the following sets of identifications is correct? (Assume that the temperature and pressure are constant).


Important Questions on Chemical Kinetics
Consider the following reactions at
(uncatalysed reaction)
(catalyst reaction)
The activation energy is lowered by the catalysed reaction. How many times is the rate of this catalysed reaction greater than that of uncatalysed reaction? (Given )
For the reaction , the rate law expression is If initial concentration of is
Calculate:
(a) Integrated form of the rate law expression
(b) Nature of plot of vs time.
(c) Half life period.
At some temperature, the rate constant for the decomposition of on a gold surface is
What is the order of the reaction? How long will it take for the concentration of to drop from .
