A small amount of calcium oxide (quick lime) is taken in a beaker. Water is slowly added to this. Which of the following observations is/are correct about this activity?
(i) The beaker becomes hot because it is an endothermic reaction.
(ii) A clear solution is obtained at the top after the reaction gets over.
(iii) This reaction is a combination reaction in which quick lime is converted into slaked lime, .

Important Questions on Chemical Reactions and Equations
Observe the given reaction carefully and identify (i), (ii), (iii) and (iv).
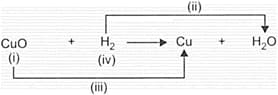
| (i) | (ii) | (iii) | (iv) | |
| (A) | Undergoes Oxidation | Oxidation | Reduction |
Undergoes reduction |
| (B) | Reducing agent | Reduction | Oxidation | Oxidising agent |
| (C) | Oxidising agent | Oxidation | Reduction | Reducing agent |
| (D) | Undergoes Reduction | Reduction | Oxidation |
Undergoes Oxidation |
Match column I with column II and select the correct answer from the given codes.
| Column I | Column II | |
| P. | Limestone is heated | Electrolysis |
| Q. | Magnesium wire is burnt in the air |
Decomposition reaction |
| R. | A white precipitate of silver chloride is formed when silver nitrate is added to sodium chloride solution | Combination reaction |
| S. | Electricity is passed through acidulated water |
Double displacement reaction |
A student wrote three statements about rancidity:
I. When fats and oils are reduced, they become rancid.
II. Chips manufacturers usually flush chips bags with oxygen to prevent rancidity.
III. Rancidity is prevented by adding substances called antioxidants to food.
Choose the correct statement(s).
Metal X is found in the earth’s crust. This metal forms a reddish-brown substance when exposed to moist air. When a blue coloured solution Y is stored in a container made of X, the solution turns green and a reddish-brown metal Z gets deposited on the container.
X, Y and Z are respectively
x and y in the given reaction are respectively.
For the given reaction, match column I with column II and mark the correct option from the codes given below.
| Column I | Column II | ||
| (a) | Oxidising agent | (i) | |
| (b) | Reducing agent | (ii) | |
| (c) | (iii) | ||
| (d) | (iv) |
