HARD
10th CBSE
IMPORTANT
Earn 100
A small piece of aluminium metal was placed in a beaker containing a greenish solution. After some time, a grey coating was observed on the aluminium piece. The greenish solution is most likely to be:
50% studentsanswered this correctly
Important Questions on Multiple Choice Questions(MCQ's)
MEDIUM
10th CBSE
IMPORTANT
A student measured the pH values of four solutions marked A, B, C and D and found them to be and respectively. The solution which is likely to be strongly acidic is
HARD
10th CBSE
IMPORTANT
Which of the following solutions should be put on a universal indicator paper so that its colour may change to green
HARD
10th CBSE
IMPORTANT
A student took solution X in a test-tube and added a few drops of universal indicator to it. The solution turned blue. On adding another solution Y to this test-tube drop-wise, the colour of solution changed to green. When a yet another solution Z was added, the solution turned yellow. Which of the following gives the correct conclusion of the student?
MEDIUM
10th CBSE
IMPORTANT
In order to study the neutralisation reaction of acid and base, a student took of dilute hydrochloric acid in a conical flask and added a few drops of phenolphthalein indicator to it. He then added dilute sodium hydroxide solution to the conical flask dropwise with a dropper while shaking the conical flask constantly. When the acid is completely neutralised by the base, the solution in conical flask will turn:
EASY
10th CBSE
IMPORTANT
In an experiment to measure the pH values of solutions, a student placed strips of universal indicator paper in four beakers containing solutions A, B, C and D. The colour of universal indicator paper in these solutions is as shown below:
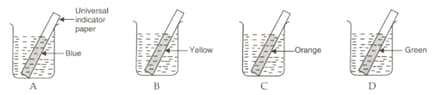
The solution having lowest pH value is :
HARD
10th CBSE
IMPORTANT
A student was given two metal strips X and Y along with colourless silver nitrate solution to perform two separate displacement reactions. When the student placed metal strip X in silver nitrate solution for a considerable time, he observed that the solution turned blue and a coating of silver metal was formed on the strip. And when the student immersed metal strip Y in silver nitrate solution for an equal time, he observed that the solution turned light green with the formation of a coating of silver metal on the strip. The correct conclusion of the student about the identity of metals X and Y is:
HARD
10th CBSE
IMPORTANT
A student was given four unknown solutions in test-tubes marked A, B, C and D and asked to test them with universal indicator solution. He observed that on putting universal indicator solution, the solutions A, B, C and D turned blue, orange, green and red respectively. The test-tube which contains sodium chloride solution is:
HARD
10th CBSE
IMPORTANT
When a student added red litmus to an aqueous solution, the red litmus turned blue. Which one of the following should be added in excess so that the change in colour is reversed?
