A student added blue litmus solution to a colourless aqueous solution. The solution turned red. Which one of the following chemicals should be added in excess so that the change in colour is reversed?

Important Questions on Multiple Choice Questions(MCQ's)
Aluminium sulphate and copper sulphate solutions were taken in two test tubes I and II respectively. Iron filings were then added to both the solutions. The four students A, B, C and D recorded their observations in the form of a table as given below.

The correct set of observations have been recorded by the student:
A student was given three solutions marked X, Y and Z, and asked to arrange them in the increasing order of their pH values. The student put two drops of each solution on three strips of universal indicator paper separately. The colours shown by the three indicator strips are as follows:
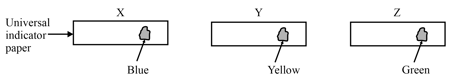
Which of the following gives the correct order of increasing pH values.
A student took four test tubes and containing Aluminium sulphate, Copper sulphate, Ferrous sulphate and Zinc sulphate solution respectively. He placed Iron strips in each one of them. After sometime, he found a brown deposit formed in test tube marked:

of Ethanoic acid was taken in each test tube- . A red litmus paper was introduced in test tube and a paper was introduced in test tube . The experiment was performed by four students and , and they reported their observations as given below:

The correct set of observations have been recorded by the student:
Four students and were asked by their teacher to arrange the set-ups to as given below and identify the gas, if any, evolved in each case.
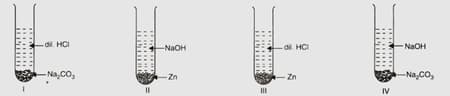
After observations, the students arrived at the interferences recorded in the following table:
| Students | ||||
| Hydrogen | Carbon dioxide | Carbon dioxide | Hydrogen | |
| Carbon dioxide | Hydrogen | No gas | Carbon dioxide | |
| Carbon dioxide | Hydrogen | Hydrogen | No gas | |
| No gas | Carbon dioxide | Carbon dioxide | Hydrogen |
The correct observations and inferences have been reported by the student:
