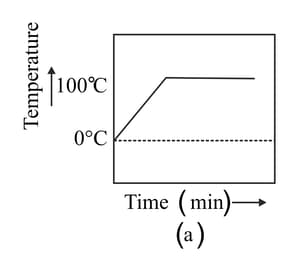
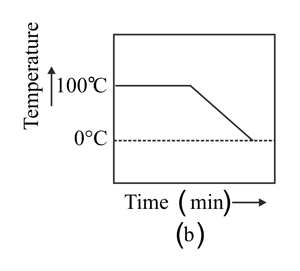
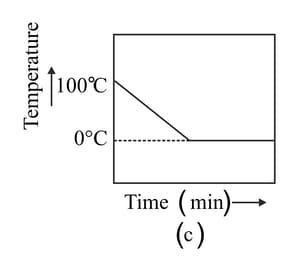
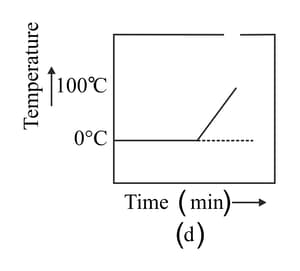
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following figures would correctly represent the result? Justify your choice.





Important Questions on Sample Paper
In the following table the mass number and the atomic number of certain elements are given:
| Element | A | B | C | D | E |
| Mass number | |||||
| Atomic number |
(A) State the pair of isobars from the above table.
(B) What would be the valency of the element C listed in the above table?
(C) Which two subatomic particles are equal in number in a neutral atom?
In a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is called the law of conservation of _____.
The following substances are added to water kept in a beaker:
(i) Sugar (ii) Milk (iii) Sand
Observe the mixtures created for stability (setting down of particles) and filterability.
Although sponge is a solid, yet we can compress it. Why?
