A student is given a solution of sodium hydroxide, hydrochloric acid, and phenolphthalein indicator. Now, to a solution of sodium hydroxide in a test tube, two drops of phenolphthalein are added. If dilute is added drop wise to the solution, what will be the colour change?

Important Questions on Acids, Bases and Salts
On adding a few drops of Universal indicator solution to three unknown colourless solutions and taken separately in three test tubes shown in the diagrams, a student observed the changes in colour as green in solution , red in solution and violet in solution . The decreasing order of of the three solutions is:

Four gas jars filled with sulphur dioxide gas were inverted into troughs of water by four students and the following observations and inference were reported:
(a) Water did not enter the gas jar and sulphur dioxide is insoluble in water.
(b) A small amount of water entered the gas jar slowly and sulphur dioxide is sparingly soluble in water.
(c) Water rushed into the gas jar and sulphur dioxide is highly soluble in water.
(d) Water did not enter the gas jar and sulphur dioxide is soluble in water.
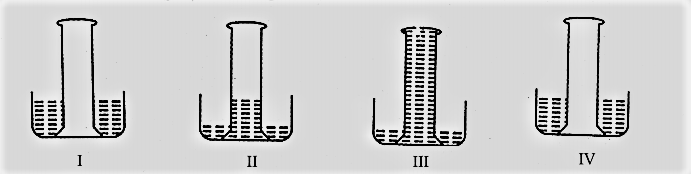
The correct set of observations and inference drawn is reported in:
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) The temperature of the solution increases
(Iii) The temperature of the solution decreases
(iii) The temperature of the solution remains the same
(iv) Salt formation takes place
Which of the following is(are) true when (g) is passed through water?
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution.
(iii) It gives both hydrogen and hydroxyl ion in the solution.
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
Which two reactants can be used to make the salt called sodium chloride?
a. chlorine
b. Sodium carbonate
c. hydrochloric acid
d. sulphuric acid
