HARD
10th CBSE
IMPORTANT
Earn 100
A student performed the following four experiments.
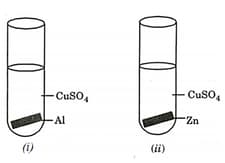
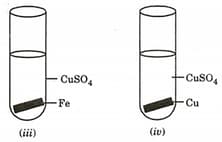
The experiments in which solid deposition on the metal plate will be observed are:


The experiments in which solid deposition on the metal plate will be observed are:
(a)(i) and (ii)
(b)(i), (ii) and (iii)
(c)(ii) and (iii)
(d)(ii), (iii) and (iv)
40% studentsanswered this correctly

Important Questions on Metals and Non-Metals
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
She recorded her observations in the following statements:
(i) No reaction occurred
(ii) Reaction occurred and aluminium sulphate was formed
(iii) Zinc is more reactive than aluminium
(iv) Aluminium is more reactive than zinc.
The correct observations are:
HARD
10th CBSE
IMPORTANT
