EASY
Earn 100
A substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as:
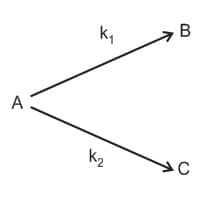
,
The percentage distribution of are
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
Consider the following reversible reaction:
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
HARD
For an elementary chemical reaction, , the expression for is:
HARD
For a first order reaction at constant volume and 300 K, the total pressure at the beginning and at time are , respectively. Initially, only A is present with concentration , and is the time required for the partial pressure of A to reach of its initial value. The correct option(s) is (are)
(Assume that all these gases behave as ideal gases)
HARD
The reaction follows first order kinetics. The pressure of a vessel containing only was found to increase from 50 mm Hg to 87.5 mm Hg in 30 min. The pressure exerted by the gases after 60 min. Will be (Assume temperature remains constant) :
MEDIUM
The rate of a first-order reaction is at second and at seconds after initiation of the reaction. The half-life period of the reaction is:
EASY
The rate of a reaction doubles when its temperature changes from . Activation energy of such a reaction will be:
MEDIUM
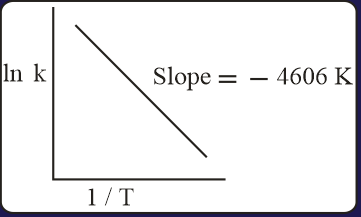
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
MEDIUM
A reaction has an activation energy of . The rate increases -fold when the temperature is increased from to . The temperature is closest to
[Gas constant, ]
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

EASY
Decomposition of exhibits a rate constant of . How many years are required for the decomposition of of into ?
EASY
, the rate equation for the reaction is given as
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
HARD
According to the Arrhenius equation,
EASY
For the equilibrium, . If the ratio of the activation energies of the forward and reverse reactions is then:
EASY
The rate constant of the reaction is mole per liter per second. If the initial concentration of A is 5 M, then the concentration of B after 20 minutes is:
MEDIUM
For the non-stoichiometry reaction, , the following kinetic data were obtained in three separate experiments, all at .
| Initial Concentration (A) | Initial Concentration (B) | Initial rate of formation of C |
|---|---|---|
The rate law for the formation of is
EASY
When initial concentration of a reactant is doubled in a reaction, its half-life period is not affected. The order of the reaction is:
EASY
The enthalpy of an elementary exothermic reaction is schematically plotted against the reaction coordinate. The plots in the presence and absence of a catalyst are shown in dashed and solid lines, respectively. Identify the correct plot for the reaction.
MEDIUM
For the reaction, products, when the concentration of and both were doubled, the rate of the reaction increased from to When the concentration of alone is doubled, the rate increased from to
Which one of the following statements is correct?
EASY
The addition of a catalyst during a chemical reaction alters which of the following quantities?
EASY
For order of reaction, Half-life period is directly proportional to

