MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
A system containing real gas changes its state form state to state .
State
State
If the change in internal energy is , calculate the change in enthalpy?
State
If the change in internal energy is , calculate the change in enthalpy?
(a)
(b)
(c)
(d)None of these
66.67% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
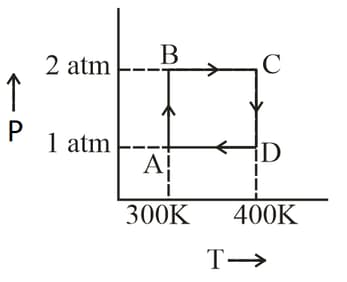
Two moles of helium gas undergo a reversible cyclic process as shown in the figure. Assuming the gas to be ideal, what is the net work involved in the cyclic process?
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
