A teacher showed his class the reaction of sodium with water. He filled a large glass container with water and put in some phenolphthalein indicator. He put some plastic screens between the container and the students.
He put on his splash-proof eye protection and used a sharp knife to cut off a very small bit of sodium from a large chunk.
He picked up the small bit using the tweezer and added it to the water.
As soon as the sodium hit the water it gave off a gas that pushed it around the surface of the water. Which gas is produced in this reaction? The reaction also gave out heat and the water turned pink. What evidence is there that sodium hydroxide was made during the reaction?



Important Questions on Materials: Metals and Non-Metals
An element X is mentioned times in the Bible, and was best known for destroying Sodom and Gomorrah. It was also known to the ancient Greeks, and burnt as a fumigant. It was mined near Mount Etna in Sicily and used for bleaching cloth and preserving wine, both of which involved burning it to form its dioxide, and allowing this to be absorbed by wet clothes or the grape juice.
The element X has several allotropes. The most common appears as yellow crystals or powder. It is a non-metal and belongs to oxygen family. This element is non-toxic but its compounds with carbon, hydrogen and oxygen are dangerous.

Identify the element X and its compounds with carbon, hydrogen and oxygen. Also mention the uses of this element.
Radha is a grade student. The teacher gave one task for her to identify the substance. The following clues are given by the teacher:
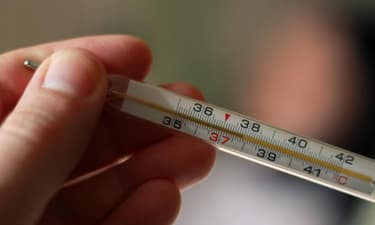
1. This substance does not make any sound at room temperature.
2. We cannot make it into a thin sheet at room temperature.
3. It is a good conductor of electricity.
4. It is used to measure the temperature.
Can you help Radha to identify the substance and mention its state?
In a laboratory, a group of students are engaged in an activity. They burnt a solid, hard, silvery-grey ribbon that they had taken. When they burnt it, it turned into a white powdery substance. So they gathered that white powdery substance in a watch glass and kept aside for later observation. Unknowingly, one of the students poured water into the watch glass. She became curious about the type of substance that is formed. So, she added a phenolphthalein indicator to the substance formed and observed that the colour of the solution was turning pink.

From the above comprehension, can you answer the below question?
a. Give the name of the silvery grey substance.
b. Write down what is formed when it is burnt, as well as the nature of the product formed.
c. What happens when it is dissolved in water ?
d. Write the chemical equation of the reaction involved when a white powdery substance is dissolved in water.
Students were doing experiments in a laboratory. Unexpectedly, one of the students poured a viscous liquid onto the copper plate, and suddenly they saw that a blue-coloured solution was forming. They knew that the liquid was an acid. To understand what happened between copper plate and acid, they collected information about types of chemical reactions, and then they learned the concept of reactivity series. Using the reactivity series, students discovered that a displacement reaction occurred in this case. The acid used is also known as the "king of chemicals" and is used as a dehydrating agent. It is also used in the manufacture of fertilizers, pigments, dyes, drugs, and explosives.

Answer the following question based on the above understanding:
1. Identify the acid and conclude whether it is concentrated or dilute.
2. What happens when this acid is poured onto a copper plate? Create word equation for the reactions involved.
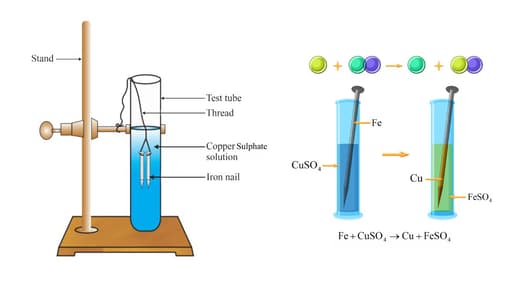
Isha and Rajiv were experimenting in the laboratory. They decided to do two different experiments to find out the reactivity of the metals.

Isha placed iron nails in a copper sulphate solution. Rajiv placed copper nails in an iron sulphate solution. Both of them found different observations. Then the teacher gave them hint that iron displaces copper from copper sulphate solution, but copper cannot displace iron from an iron sulphate solution. Now find out Why? Name the type of reaction that takes place.
Right side of the periodic table represents non-metals which are less in numbers than metals. Metals and non-metals have different chemical properties. They both react with oxygen to give oxides, but they have distinguished properties.
A non-metal oxide is acidic in nature because when they react with water, they form acids. One such non-metal oxide is sulphur dioxide.
Sulphur oxide's acidic nature is justified when it is reacted with water. It gives sulphurous acid.
An acid when reacts with a base forms a salt. This is the another way to identify the acidic nature of a non-metallic oxide. These oxides when reacts with a base, acts as an acid to give salts.
- A triatomic gas is released during the fermentation of sugars in bread. It is also produced by combustion of wood, peat and other organic materials and fossil fuels such as coal, petroleum and natural gas. Name the gas formed and justify its acidic nature on the basis of above established methods.
A brown shiny metal known as cuprum is used since ancient times. It easily gets corroded when exposed to air. The metal is used in buildings, usually for roofing, and oxidises to form a green verdigris like in the case of 'Statue of Liberty'. The Statue of Liberty is a colossal neoclassical sculpture on Liberty Island in New York Harbour in New York City, in the United States. The metal statue, a gift from the people of France to the people of the United States, was designed by French sculptor Frédéric Auguste Bartholdi and its metal framework was built by Gustave Eiffel. The statue was dedicated on October .
The image for then and now of the statue is shown below.

Identify the metal and mention its two uses. What is the green layer formed over the statue?
A class 8 student Rajesh accidentally touched a wire whose outer cover was damaged. He got a shock as the current was running through the wire, but fortunately no major mishap happened. What material do you think, are wires made of? And why do you think coated wire protect us from getting a shock?

