A thermally insulated vessel is divided into two parts by a heat-insulating piston which can move in the vessel without friction. The left part of the vessel contains one mole of an ideal monatomic gas, and the right part is empty. The piston is connected to the right wall of the vessel through a spring whose length in free state is equal to the length of the vessel (Fig.).
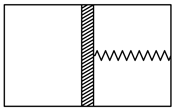
Determine the heat capacity of the system, neglecting the heat capacities of the vessel, piston, and spring.


Important Questions on Heat and Molecular Physics
Let us suppose that a planet of mass and radius is surrounded by an atmosphere of constant density, consisting of a gas of molar mass .
Determine the temperature of the atmosphere on the surface of the planet if the height of the atmosphere is .
It is known that the temperature in the room is when the outdoor temperature is , and when the outdoor temperature is .
Determine the temperature of the radiator heating the room.
A space object has the shape of a sphere of radius . Heat sources ensuring the heat evolution at a constant rate are distributed uniformly over its volume. The amount of heat liberated by a unit surface area is proportional to the fourth power of thermodynamic temperature.
In what proportion would the temperature of the object change if its radius decreased by half?
A heat exchanger of length consists of a tube of cross-sectional area with another tube of cross-sectional area passing through it (Fig). The walls of the tubes are thin. The entire system is thermally insulated from the ambient. A liquid of density and specific heat is pumped at a velocity through the tubes. The initial temperatures of the liquid in the heat exchanger are and respectively.
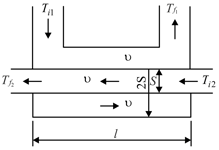
Determine the final temperatures and of the liquid in the heat exchanger if the liquid passes through the tubes in the counterflow, assuming that the heat transferred per unit time through a unit area element is proportional to the temperature difference, the proportionality factor being . The thermal conductivity of the liquid in the direction of its flow should be neglected.
A closed cylindrical vessel of base area contains a substance in the gaseous state outside the gravitational field of the Earth. The mass of the gas is and its pressure is such that , where is the saturated vapour pressure at a given temperature. The vessel starts moving with an acceleration directed along the axis of the cylinder. The temperature is maintained constant.
Determine the mass of the liquid condensed as a result of motion in the vessel.
