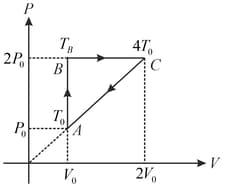
A thermodynamic process of one mole ideal monoatomic gas is shown in figure. The efficiency of cyclic process will be:

Important Questions on Thermodynamics
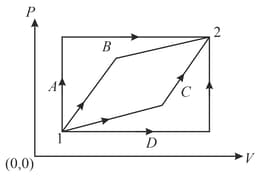
One mole of a gas is subjected to two process and one after the other as shown in the figure. is represented by, . We can conclude that (where, temperature, work done by gas, volume and internal energy),
An ideal gas is taken from state to state through optional path and , as shown in the diagram. Let and represent the heat supplied, work done and change in internal energy of the gas, respectively. Then,
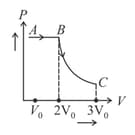
One mole of an ideal gas requires heat to raise the temperature by when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by then heat required is [given gas constant .]
Assertion: Equal volumes of monatomic and polyatomic gases are adiabatically compressed separately to equal compression ratio . Then polyatomic gas will have greater final volume.
Reason: Among ideal gases, molecules of a monatomic gas have the smallest number of degrees of freedom.
Two samples and are initially kept in the same state. Sample is expanded through an isothermal process whereas sample through an adiabatic process up to the same final volume. Let and be the final pressures of the samples and , respectively, then,
