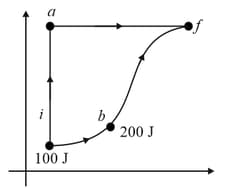
A thermodynamic system is taken from an initial state with internal energy to the final state along two different paths and as schematically shown in the figure. The work done by the system along the paths and are and respectively. The heat supplied to the system along the path and are and respectively. If the internal energy of the system in the state is and , then the ratio is


Important Questions on The First Law of Thermodynamics
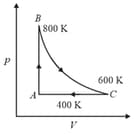
One mole of diatomic ideal gas undergoes a cyclic process as shown in figure. The process is adiabatic. The temperature at and are , and respectively. Choose the correct statement.
One mole of monoatomic ideal gas is taken along two cyclic processes and as shown in the diagram.
The processes involved are purely isochoric, isobaric, isothermal or adiabatic.
Match the paths in List I with the magnitudes of the work done in List II and select the correct answer using the codes given below the lists.
| Column I | Column II | ||
| P. | |||
| Q. | |||
| R. | |||
| S. |
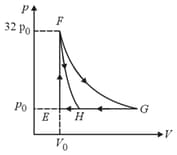
The shown diagram represents the thermodynamic cycle of an engine, operating with an ideal mono atomic gas. The amount of heat, extracted from the source in a single cycle is
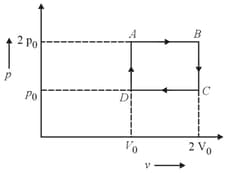
An ideal gas is taken around as shown in the figure of diagram. The work done during a cycle is
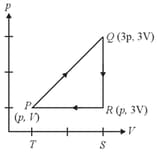
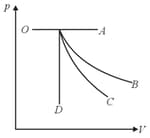
A graph of pressure versus volume for an ideal gas for different processes is as shown. In the graph, curve represents