MEDIUM
Physics
IMPORTANT
Earn 100
A vessel contains mole gas at a temperature . An identical vessel contains one mole of He gas at a temperature . Find the ratio of their pressure ( to ).
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
HARD
Physics
IMPORTANT
Given figure shows a horizontal cylindrical container of length , which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in the figure. The temperature of left part of cylinder is and that on right part is . Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator (in ) after a long time when gases on the two parts of cylinder are in thermal equilibrium.
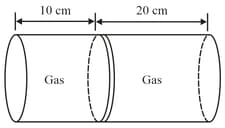
EASY
Physics
IMPORTANT
One kilogram of a diatomic gas is at a pressure of . The density of the gas is . What is the energy of the gas due to its thermal motion?
HARD
Physics
IMPORTANT
Consider a spherical shell of radius at a temperature . The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume and pressure . If the shell now undergoes an adiabatic expansion the relation between and is :
EASY
Physics
IMPORTANT
A real gas behaves like an ideal gas if its
MEDIUM
Physics
IMPORTANT
A mixture of moles of helium gas (atomic mass) and mole of argon gas (atomic mass) is kept at in a container. The ratio of the rms speed is
EASY
Physics
IMPORTANT
A vessel contains a mixture of mole of oxygen and 2 moles of nitrogen at . Find the ratio of average rotational kinetic energies per molecule to per molecule.
MEDIUM
Physics
IMPORTANT
A vessel of volume contains an ideal gas at and . The gas is allowed to leak till the pressure falls to . Calculate the amount of the gas (in moles) leaked assuming that the temperature remains constant? (Given )
MEDIUM
Physics
IMPORTANT
Oxygen is filled in a closed metal jar of volume at a pressure of and temperature . The jar has a small leak in it. The atmospheric pressure is and the atmospheric temperature is . Find the mass (in ) of the gas that leaks out by the time the pressure and the temperature inside the jar equalise with the surrounding.
