MEDIUM
Physics
IMPORTANT
Earn 100
A vessel contains a mixture of of nitrogen and of carbon dioxide at temperature . If the pressure of the mixture is, its density is (gas constant )
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
Physics
IMPORTANT
A spherical balloon contains air at temperature and pressure . The balloon material is such that the instantaneous pressure inside is proportional to the square of the diameter. When the volume of the balloon doubles as a result of heat transfer, the expansion follows the law
MEDIUM
Physics
IMPORTANT
The ratio of pressure of the same gas in two containers is where and are the number of moles and and are respective temperatures. If the two containers are now joined find the ratio of pressure to the temperature:
MEDIUM
Physics
IMPORTANT
On an isothermal process, there are two points and at which pressures and volumes are and respectively. If and are connected by a straight line, find the pressure at a point on this straight line at which temperature is maximum:
MEDIUM
Physics
IMPORTANT
In the previous problem, the temperature of the isothermal process is how much lower the maximum temperature:
MEDIUM
Physics
IMPORTANT
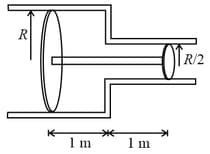
Two cylinders fitted with pistons and placed as shown, connected with string through a small tube of negligible volume, are filled with a gas at pressure and temperature . The radius of smaller cylinder is half of the other. If the temperature is increased to , find the pressure, if the piston of bigger cylinder moves towards left by metre ?
MEDIUM
Physics
IMPORTANT
Two identical containers and having same volume of an ideal gas at the same temperature have mass of the gas as and respectively. . The gas in each cylinder expand isothermally to double its volume. If the change in pressure in is , find the change in pressure in :
MEDIUM
Physics
IMPORTANT
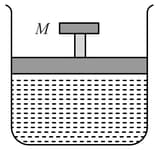
A cylinder containing an ideal gas is in vertical position and has a piston of mass M that is able to move up or down without friction (Figure). If the temperature is increased,
MEDIUM
Physics
IMPORTANT
mole of gas is contained in a box of volume at . The gas is heated to a temperature of and the gas gets converted to a gas of hydrogen atoms. The final pressure would be (considering all gases to be ideal)
