EASY
Earn 100
A vessel of volume 8.3 × 10–3 m3 contains an ideal gas at temperature 27ºC and pressure 200 kPa. The gas is allowed to leak till the pressure falls to 100 kPa and temperature increases to 327ºC. What is the amoutn of gas in moles will be leaked out?
Important Questions on Kinetic Theory of Gases
EASY
MEDIUM
EASY
HARD
EASY
EASY
MEDIUM
EASY
HARD
EASY
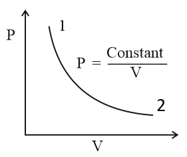
Out of the following which one correctly represents the diagram?
HARD
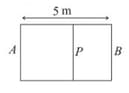
The left compartment is filled with a given mass of an ideal gas of molar mass while the right compartment is filled with an equal mass of another ideal gas of molar mass at same temperature. What will be the distance of from the left wall when equilibrium is established?
EASY
HARD
EASY
MEDIUM
Different curves in the figure show the behaviour of gases
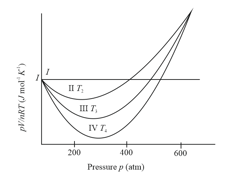
(i) Curve I represent ideal gas behaviour
(ii) Curves II, III and IV also represents ideal gas behaviour at different temperatures and
(iii) Curves II, III and IV represents behaviour of a real gas at different temperatures and
(iv)
(v)
The correct statements are
EASY
EASY
HARD
(Atmospheric pressure = of Hg)
EASY
MEDIUM
( is universal gas constant and is the acceleration due to gravity)

