EASY
Earn 100
According to Charles’s law, .
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Thermal Physics
MEDIUM
EASY
A perfect gas at is heated at constant pressure so as to double its volume. The increase in temperature of the gas will be
MEDIUM
EASY
EASY
MEDIUM
Reason: On increasing the pressure the temperature of gas increases at constant volume.
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
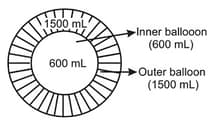
MEDIUM
MEDIUM
EASY
Volume-temperature graph at atmospheric pressure for a monoatomic gas ( is
EASY
MEDIUM
MEDIUM
HARD
EASY

