EASY
CUET (UG)
IMPORTANT
Earn 100
According to Einstein's photoelectric equation, the graph between the kinetic energy of photoelectrons ejected and the frequency of incident radiation is
(a)
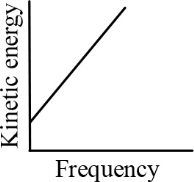

(b)
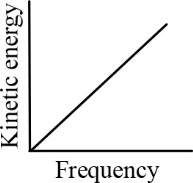

(c)
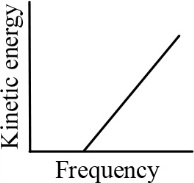

(d)
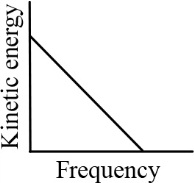

50% studentsanswered this correctly

Important Questions on Dual Nature of Radiation and Matter
EASY
CUET (UG)
IMPORTANT
Which one of the following is true in photoelectric emission?
EASY
CUET (UG)
IMPORTANT
Photoelectric effect takes place in element . Its work function is and threshold wavelength is . Another element has work function of . Then, find out the wavelength that can produce photoelectric effect in .
EASY
CUET (UG)
IMPORTANT
The idea of the quantum nature of light has emerged in an attempt to explain
EASY
CUET (UG)
IMPORTANT
Find the maximum kinetic energy of the photo electron liberated from the surface of lithium by electromagnetic radiation whose electric component varies with time as
, where is a constant, and , work function of lithium,
EASY
CUET (UG)
IMPORTANT
The number of photons of frequency present in energy is:
EASY
CUET (UG)
IMPORTANT
Photoelectric effect experiments are performed using three different metal plates p, q and r having work functions and respectively, A light beam containing wavelength of and with equal intensities illuminates each of the plates. The correct graph for the experiment is, see Fig.
EASY
CUET (UG)
IMPORTANT
Sodium and copper have work function and respectively. Then, the ratio of the wavelength is nearest to:
MEDIUM
CUET (UG)
IMPORTANT
A beam of light of intensity is normally incident on a plate of area . If plate absorbs fraction of incident light and reflects rest of it back, then the force exerted by the incident light on the plate is given by [=speed of light]
