MEDIUM
Earn 100
According to MO theory which of the lists ranks the nitrogen species in terms of increasing bond order?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
HARD
Decreasing order of stability of and is:
HARD
HARD
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
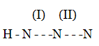
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
[Resonance energy of benzene
Enthalpy of hydrogenation of cyclohexene ]
EASY
Assertion: In the bonding molecular orbital (MO) of electron density is increased between the nuclei.
Reason: The bonding MO is , which shows destructive interference of the combining electron waves.
MEDIUM
The molecule/molecules that has/have delocalised lone pair(s) of electrons is/are
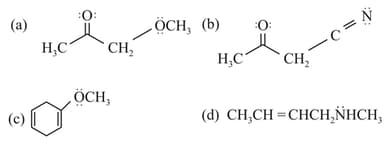
MEDIUM
EASY
EASY
EASY
MEDIUM
(i)
(ii)
(iii)
Among the following, sigma bond alone is present in -
MEDIUM
Resonance in carbonate ion is
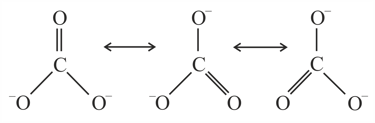
Which of the following is true?
MEDIUM
MEDIUM

