According to law of mass action, the rate at which a substance reacts is proportional to its (or molar concentration).
Important Questions on Equilibrium
.....(1)
.....(2)
The relation between and is:
(At )
For the following reaction, equilibrium constant are given:
The equilibrium constant for the reaction, is:
(assuming ideality)
Sucrose Glucose Fructose
If the equilibrium constant is at , the value of at the same temperature will be:
When and are compared at It is found that
When equal volumes of and solutions are mixed, the equilibrium concentration of is found to be The value of is __________ (Answer should be given to the nearest integer value).
The value of for the reaction:
is Calculate the value of in .
Consider the reaction at . At the time , the temperature of the system was increased to and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of was maintained at bar. Given below is the plot of the partial pressure of with time. What is the ratio of the standard Gibbs energy of the reaction at to that at ?
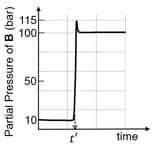
Give your answer by multiplying with 100 and rounding off to nearest integer.
The equilibrium constant () of the reaction:
, will be:
A solid kept in an evacuated sealed container undergoes decomposition to form a mixture of gases at temperature .
The equilibrium pressure is in this vessel. for this reaction is?

