According to second law of thermodynamics :
Important Questions on Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Which of the following relations is correct?
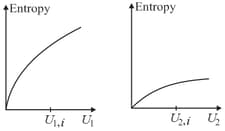
Graphs below show the entropy versus energy of two systems and at constant volume. The initial energies of the systems are indicated by and respectively. Graphs are drawn to the same scale. The systems are then brought into thermal contact with each other. Assume that, at all times the combined energy of the two systems remains constant. Choose the most appropriate option indicating the energies of the two systems and the total entropy after they achieve the equilibrium.
Step It is first compressed adiabatically from volume to
Step then expanded isothermally to volume
Step then expanded adiabatically to volume
Step then compressed isothermally to volume If the efficiency of the above cycle is then is
(Specific heat of water liquid and water vapour are and ; heat of liquid fusion and vaporization of water are and , respectively). ( )
An engine operating between the boiling and freezing points of water will have
A. Efficiency more than .
B. Efficiency less than the efficiency of a Carnot engine operating between the same two temperatures.
C. Efficiency equal to .
D. Efficiency less than .
Choose the correct answer from the options given below
What will be the and values for the given cell having at and to be and respectively?
The cell: (Pt) (Given: Coulombs)
For the following reaction
and . What is the value of at ?

