HARD
JEE Advanced
IMPORTANT
Earn 100
According to the Arrhenius equation,
(a)A high activation energy usually implies a fast reaction
(b)Rate constant increase with increase in temperature. This is due to a greater number of collisions whose energy exceeds the activation energy
(c)Higher the magnitude of activation energy, stronger is the temperature dependence of the rate constant
(d)The pre-exponential factor is a measure of the rate at which collisions occur, irrespective of their energy.
25% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
JEE Advanced
IMPORTANT
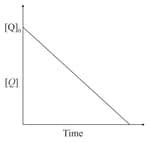
In the reaction,
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is: