EASY
Earn 100
According to the Bohr Bury rule, the maximum number of electrons in a particular orbit is given by the formula . Here, the value of a will be .
50% studentsanswered this correctly
Important Questions on Structure of The Atom
EASY
MEDIUM
MEDIUM
MEDIUM
The schematic atomic structure of four elements is given below. Observe and choose the right statement.
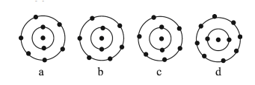
EASY
MEDIUM
An isoelectronic species are
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
Nitrogen (atomic number ) and phosphorus (atomic number ) belong to group of the Periodic Table. Write the electronic configuration of these two elements. Which one of these will be more electronegative? Why?
EASY
EASY
MEDIUM
EASY
EASY
HARD
What do you understand by the term electronic configuration? State Bohr-Bury's scheme of electronic configuration.
EASY
MEDIUM
MEDIUM

