According to the assumptions made in the kinetic theory of gases, when two molecules of a gas collide with each other then

Important Questions on Behaviour of Perfect Gas and Kinetic Theory
Consider a sample of oxygen behaving like an ideal gas. At , the ratio of root-mean-square (RMS) velocity to the average velocity of the gas molecule would be :
(Molecular weight of oxygen is )
Assertion: An ideal gas is enclosed within a container fitted with a piston. When volume of this enclosed gas is increased at constant temperature, the pressure exerted by the gas on the piston decreases.
Reason: In the above situation, the rate of molecules striking the piston decreases. If the rate ạt which molecules of a gas having same average speed striking a given area of the wall decreases, the pressure exerted by gas on the wall decreases.
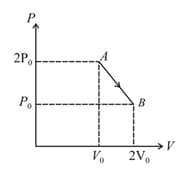
of an ideal gas undergoes a process as shown in the figure. The maximum temperature of the gas during the process will be