Acetic acid forms a dimer in the gas phase:
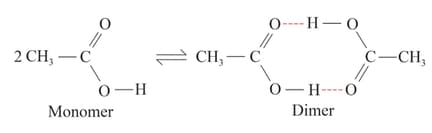
The dimer is held together by two hydrogen bonds with a total strength of per mole of the dimer. At , the equilibrium constant for the dimerisation is (pressure in ). What is for the reaction? Assume that does not vary with temperature.


Important Questions on Thermodynamics
When of carbon reacted with oxygen to form and at and constant pressure, of heat was liberated and no carbon remained. Calculate the mass of oxygen that reacted. Given,
Answer correct up to one place of decimal.
Assuming that of the heat is useful, how many of water, at , can be heated to by burning of methane at
Answer after rounding off to the nearest integer value.
The thermochemical equation for the dissociation of hydrogen gas into atoms may be written as
What is the ratio of the energy yield on combustion of hydrogen atoms to steam to the yield on combustion of an equal mass of hydrogen molecules to steam?
Answer correct up to one significant value.
Calculate the value of for the reaction: at . The standard enthalpy of formation of is and the standard entropies of and gases are , respectively.
Round off your answer to the nearest integer.
In a fuel cell, methanol is used as a fuel and oxygen gas is used as an oxidizer. The reaction is .
At , the standard Gibbs energies of the formation for and are and , respectively. If the standard enthalpy of combustion of methanol is , the efficiency of the fuel cell will be:
