EASY
Earn 100
Alkyl iodide reacts with NaCN to give alkyl cyanide and small amount of alkyl isocyanide. Formation of these two products is due to the
(a)Ionic character of NaCN
(b)Nucleophilic character of cyanide ion
(c)Ambident character of cyanide ion
(d)Electrophilic character of cyanide
50% studentsanswered this correctly
Important Questions on Halogen Derivatives
MEDIUM
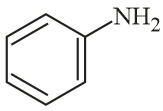
The increasing order of reactivity of the following compounds towards reaction with alkyl halides directly is:
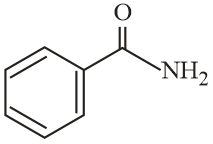
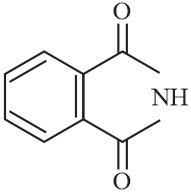
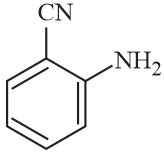
MEDIUM
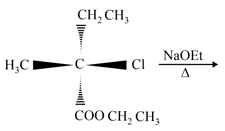
The major product of the following reaction is:
EASY
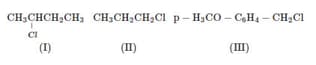
EASY
a.
b.
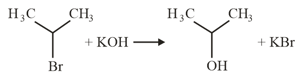
c.
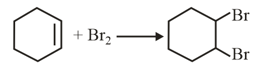
Which of the following statements is correct?
MEDIUM
MEDIUM
MEDIUM
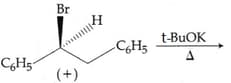
MEDIUM
EASY
This reaction will be the fastest in
EASY
HARD
The major product of the following reaction is:
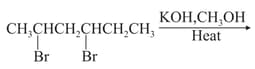
MEDIUM
MEDIUM
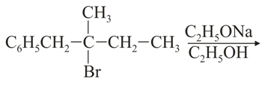
EASY
MEDIUM
(1)
(2)
(3)
is
MEDIUM
HARD
EASY
The reagent required for the following two step transformation are
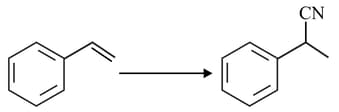
MEDIUM
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is :
MEDIUM
The major product of the following reaction is: