MEDIUM
Earn 100
All metals react with base to form corresponding salt and hydrogen. (True/False)
(a)True
(b)False
100% studentsanswered this correctly
Important Questions on Acids, Bases and Salts
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
EASY
What happens when: (write chemical equation only).
(ii) Sulphuric acid reacts with caustic soda
EASY
What happens when: (write chemical equation only).
(i) Zinc reacts with dilute sulphuric acid
MEDIUM
HARD
The salt solution which does not react with ammonium hydroxide is:
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
Which gas is produced during the reaction in the test tube? How does this gas react with calcium hydroxide/lime water?
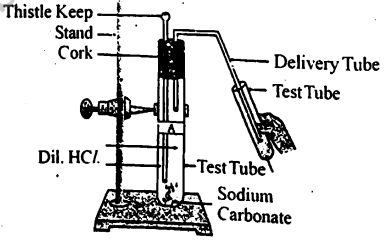
EASY
EASY
MEDIUM

