MEDIUM
10th ICSE
IMPORTANT
Earn 100
Aluminium carbide reacts with water according to the following equation:
Calculate the volume of methane measured at STP from of aluminium carbide by an excess of water.

Important Questions on Practice Paper-6
MEDIUM
10th ICSE
IMPORTANT
The total number of elements present in the third period is
MEDIUM
10th ICSE
IMPORTANT
Arrange the elements of period in the increasing order of their atomic size.
EASY
10th ICSE
IMPORTANT
Inert gases have zero valencies. Why?
MEDIUM
10th ICSE
IMPORTANT
How does ionisation potential differ in group and period?
EASY
10th ICSE
IMPORTANT
From the following formulae:
Choose one having the following description.
An acid salt.
MEDIUM
10th ICSE
IMPORTANT
From the following formulae:
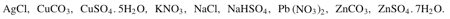
Choose one having the following description.
An insoluble chloride.
MEDIUM
10th ICSE
IMPORTANT
From the following formulae:
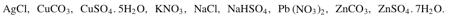
Choose one having the following description.
Changes from blue to white on heating with conc.
MEDIUM
10th ICSE
IMPORTANT
From the following formulae:
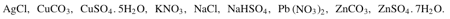
Choose one having the following description.
Changes from green to black on heating.
