EASY
NEET
IMPORTANT
Earn 100
Among , the compounds with the greatest and the least ionic character, respectively are
(a) and
(b) and
(c) and
(d) and
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
The correct order of increasing covalent character is:
EASY
NEET
IMPORTANT
The volatility of HF is low because of:
EASY
NEET
IMPORTANT
The type of molecular force of ättraction present in the following compound is
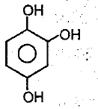
EASY
NEET
IMPORTANT
Which of the following substances does not exhibit H-bonding with water?
EASY
NEET
IMPORTANT
When metals combine with non-metals, the metal atom tends to:
EASY
NEET
IMPORTANT
The lattice energy of sodium chloride crystal is the energy released when one mole of is formed from:
EASY
NEET
IMPORTANT
Which does not favour the formation of ionic compound:
EASY
NEET
IMPORTANT
Octet configuration cannot be achieved through:
