MEDIUM
JEE Main
IMPORTANT
Earn 100
Among solids, the highest melting point is exhibited by
(a)covalent solids.
(b)ionic solids.
(c)pseudo solids.
(d)molecular solids.
12.5% studentsanswered this correctly

Important Questions on Solid State
MEDIUM
JEE Main
IMPORTANT
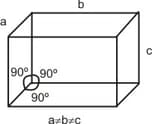
The unit cell with the following structure refers to______crystal system.
EASY
JEE Main
IMPORTANT
The crystal system of a compound with unit cell dimensions and is
EASY
JEE Main
IMPORTANT
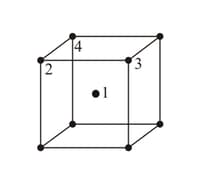
In a bcc unit cell, atoms are numbered as shown below. The atoms touching each other are:
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
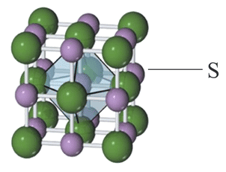
For the structure given below, the site marked as is a:
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
