EASY
JEE Main
IMPORTANT
Earn 100
Among the following, the set of parameters that represents path functions, is:
i)
ii)
iii)
iv)
(a) and
(b) and
(c) and
(d) and
58.41% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
An ideal gas undergoes isothermal expansion at constant pressure. During the process:
EASY
JEE Main
IMPORTANT
A gas undergoes change from state to state . In this process, the heat absorbed and work done by the gas is , respectively. Now gas is brought back to by another process during which of heat is evolved. In this reverse process of .
MEDIUM
JEE Main
IMPORTANT
For the reaction,
and are, respectively, and at 298 K. The equilibrium constant for the reaction at 298 k is:
EASY
JEE Main
IMPORTANT
A reaction at 1 bar is non-spontaneous at low temperature but becomes spontaneous at high temperature. Identify the correct statement about the reaction among the following:
MEDIUM
JEE Main
IMPORTANT
The standard enthalpy of formation of is . If bond enthalpy of is and that of is , the average bond enthalpy of bond in is :
MEDIUM
JEE Main
IMPORTANT
At constant volume, of an ideal gas when heated from to changes its internal energy by The molar heat capacity at constant volume is ________
MEDIUM
JEE Main
IMPORTANT
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.
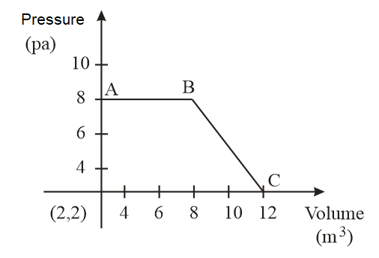
MEDIUM
JEE Main
IMPORTANT
moles of an ideal gas at are allowed to undergo reversible compression till its temperature becomes If calculate and for the process.
