EASY
Earn 100
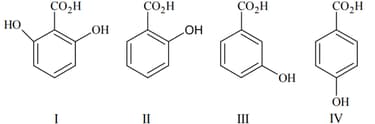
Among the following oxoacids, the correct decreasing order of acid strength is :
50% studentsanswered this correctly
Important Questions on Principles of Organic Chemistry
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
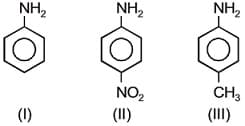
The increasing basicity order of the following compounds is:
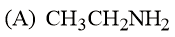
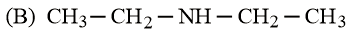
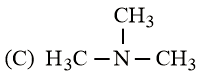
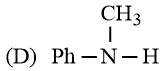
EASY
MEDIUM

EASY
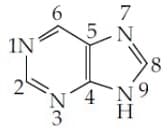
MEDIUM
HARD
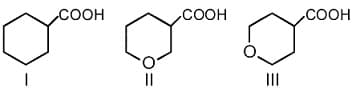
EASY
MEDIUM
MEDIUM
EASY
. Ethanol . Acetic Acid . Phenol . Acetonitrile
follows the order
HARD
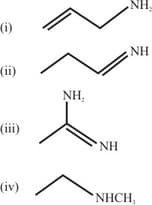
HARD
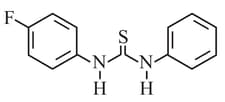
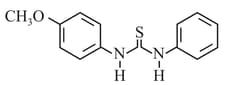
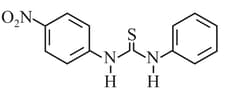
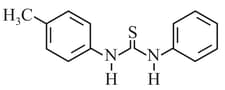
HARD
What is the order of basicity among the following compounds?
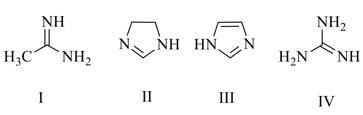
EASY
MEDIUM
HARD