MEDIUM
Earn 100
Among the following reduction processes, what is correct increasing order of reducing power of the metals?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Redox Reactions and Electrochemistry
EASY
Given the standard half-cell potentials of the following as
Then the standard e.m.f. of the cell with the reaction is
HARD
The standard reduction potential for is Calculate the reduction potential at for the above couple.
MEDIUM
Identify the reaction from following having top position in electrochemical series according to their standard electrode potential.
EASY
In a electrochemical cell, the reaction will be feasible when
MEDIUM
Consider the following standard electrode potentials ( in volts) in aqueous solution:
Based on these data, which of the following statements is correct?
EASY
The standard emf of - voltaic cell is
MEDIUM
Given that the standard potentials of and are and respectively, the of is:
MEDIUM
Four metals and have standard electrode potential as and respectively. The most reducing metal is
HARD
Given below are the half - cell reactions :
The for will be :
MEDIUM
Calculate the standard cell potential (in V) of the cell in which the following reaction takes place:
Given that
EASY
The standard emf of the cell is If the standard reduction potential of is the standard oxidation potential of is
MEDIUM
Four successive members of the first row of transition elements are listed below with atomic numbers. Which one of them is expected to have the highest value?
MEDIUM
What will be the oxidation potential for the following hydrogen half cell at bar pressure and temperature?
EASY
In an electrochemical cell, anode and cathode are respectively
MEDIUM
In which metal container, the aqueous solution of can be stored?
EASY
The hydrogen ion concentration in a standard hydrogen electrode is
EASY
What will be the half-cell potential of a hydrogen electrode acting as an anode and dipped in a solution of
EASY
Standard hydrogen electrode (SHE) is a
MEDIUM
A variable, the opposite external potential is applied to the cell , of potential . When and , respectively electrons flow from
HARD
Consider the change in oxidation state of Bromine corresponding to different emf values, as shown in the diagram below:
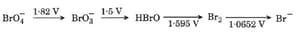
Then, what is the species undergoing disproportionation?

