HARD
JEE Main
IMPORTANT
Earn 100
Among the following structures, which will show the most stable enamine formation?
(Where is )
(a)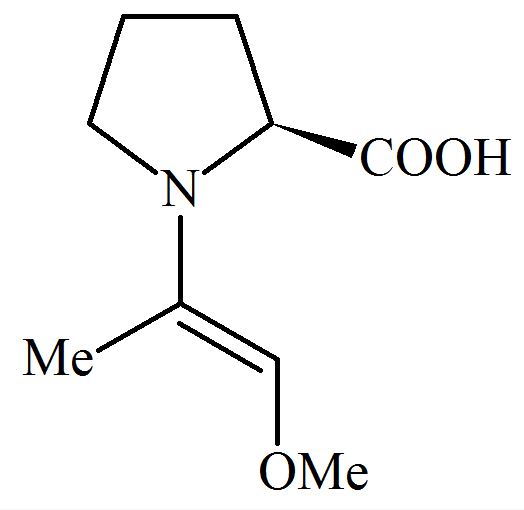
(b)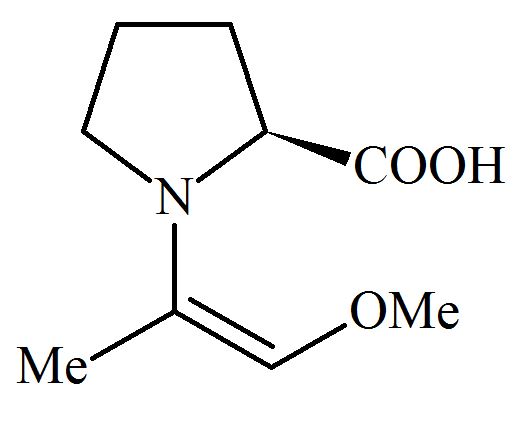
(c)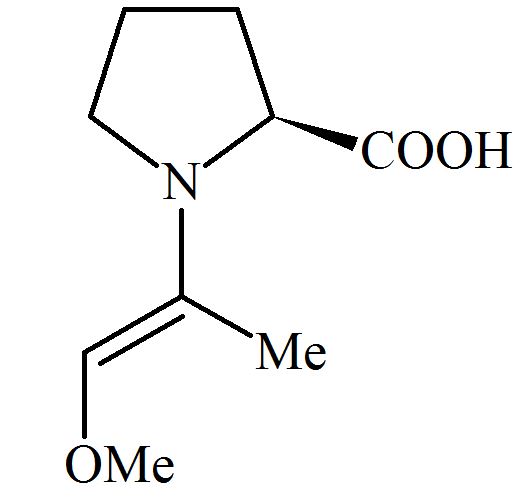
(d)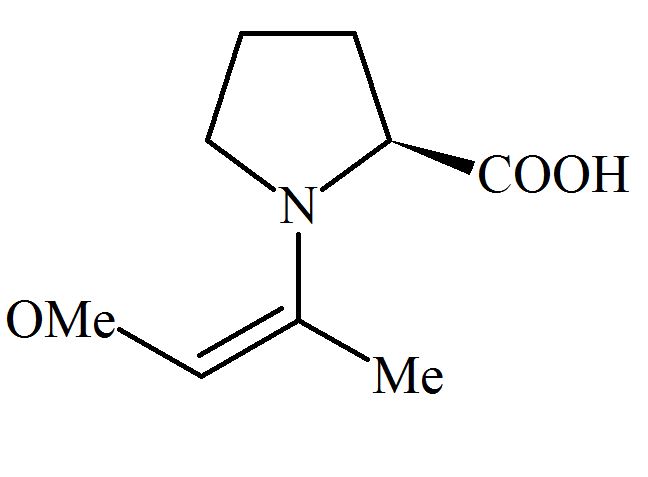
19.23% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
JEE Main
IMPORTANT
Which of the following is structure of a separating funnel?
MEDIUM
JEE Main
IMPORTANT
of an organic compound containing chlorine gave of silver chloride in Carius estimation. The percentage of chlorine present in the compound is [in nearest integer]
(Given: Molar mass of is and that of is )
MEDIUM
JEE Main
IMPORTANT
Which of the following structures are aromatic in nature?
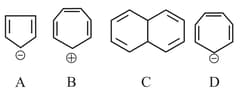
MEDIUM
JEE Main
IMPORTANT
In the estimation of bromine, of an organic compound gave of silver bromide. The percentage of bromine in the given compound is______ (nearest integer)
(Relative atomic masses of and are and , respectively).
MEDIUM
JEE Main
IMPORTANT
Given below are two statements.
Statement I : Phenols are weakly acidic.
Statement II : Therefore they are freely soluble in solution and are weaker acids than alcohols and water.
Choose the most appropriate option
MEDIUM
JEE Main
IMPORTANT
Kjeldahl's method was used for the estimation of nitrogen in an organic compound. The ammonia evolved from of the compound neutralised of solution. The percentage of nitrogen in the compound is (Nearest integer)
HARD
JEE Main
IMPORTANT
Which of the following carbocations is most stable:
HARD
JEE Main
IMPORTANT
While estimating the nitrogen present in an organic compound by Kjeldahl's method, the ammonia evolved from of the compound neutralized of . The percentage of nitrogen present in organic compound is _____ .
