Among the following the number of state variable is
Internal energy
Volume
Heat
Enthalpy

Important Questions on Thermodynamics
(Enthalpy of neutralisation and Specific heat of water ) (Neglect heat capacity of flask)
When coal of purity is allowed to burn in presence of insufficient oxygen, of carbon is converted into ' ' and the remaining is converted into ''.
The heat generated when of coal is burnt is
For independent process at .
| Process | ||
The number of non-spontaneous process from the following is _____ .
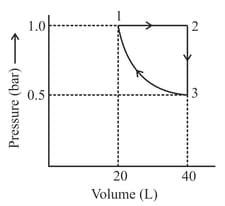
One mole of an ideal monoatomic gas is subjected to changes as shown in the graph. The magnitude of the work done (by the system or on the system) is _______ J (nearest integer)
Given : log 2 = 0.3, ln 10 = 2.3
An athlete is given of glucose for energy. This is equivalent to of energy. The of this energy gained is utilized by the athlete for sports activities at the event. In order to avoid storage of energy, the weight of extra water he would need to perspire is (Nearest integer) Assume that there is no other way of consuming stored energy.
Given : The enthalpy of evaporation of water is
Molar mass of are $12.1$ and .
L of is produced on complete combustion of gaseous mixture of ethene and methane at and atm. Heat evolved during the combustion process is
Given
.
(A)
(B)
(C)
(D)
Choose the most appropriate answer from the options given below :
