EASY
Earn 100
Amongst the following statements, select the set having statements which was proposed by Dalton.
(1) All the atoms of a given element have identical properties including identical mass. Atoms of different elements differ in mass.
(2) When gases combine or reproduced in a chemical reaction they do so in a simple ratio by volume provided all gases are at the same T & P
(3) Chemical reactions involve reorganization of atoms. These are neither created nor destroyed in a chemical reaction.
(4) Matter consists of indivisible atoms
(a)(1), (2), (3)
(b)(1), (3), (4)
(c)(1), (2), (4)
(d)(1), (2), (3), (4)
68.75% studentsanswered this correctly
Important Questions on Structure of Atom
EASY
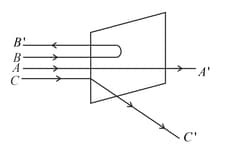
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
Which of the following statements is correct ?
EASY
MEDIUM
EASY
(At. No. Z = )
EASY
MEDIUM
EASY
EASY
EASY
(Ignore radiation due to motion of electric charge)
EASY
According to Rutherford's experiments, the size of nucleus is about :
MEDIUM
MEDIUM
MEDIUM
EASY

