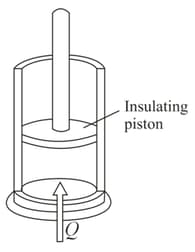
An adiabatic vertical cylinder fitted with and adiabatic frictionless piston contains moles of an ideal diatomic gas. The initial volume and temperature of the gas are and , respectively, with the piston fixed tightly. The atmospheric pressure is . If the piston be released, find the equilibrium temperature and volume of the gas, if the heat capacity of the cylinder and piston is negligibly small. Weight of the piston and cross-sectional area of the cylinder are and . respectively.


Important Questions on Thermodynamics
Four moles of an ideal gas (monatomic) at pressure and temperature is isothermally expanded to twice its initial volume. The gas is then compressed at constant pressure to its original volume. Finally, the gas is compressed at constant volume to its original pressure .
Sketch the and diagrams for the process.
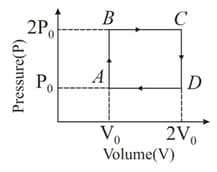
Find the efficiency of the thermodynamic cycle shown in figure, for an ideal diatomic gas.
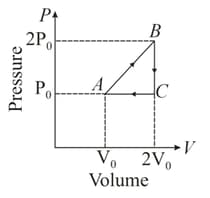
The given figure shows the indicator diagram corresponding to moles of an ideal gas taken along the cyclic process . Find the efficiency of the cycle.